THE EXPERTS IN SCIENTIFIC COMMUNICATION BEHIND THE MARKET LEADERS IN LIFE SCIENCES
We help medical device, biotech, and pharmaceutical companies
scientifically validate their products and speed up their delivery to the clinic and patients. Innovative products demand more than just brilliant ideas – they require robust evidence, well-defined study strategies, and powerful communication.
Faster to market acceptance
Become clinical standard early on. Maximize the time advantage. Those who reach milestones faster sell earlier, gain more visibility – and often become the new standard of care.
Evidence that drives sales
Position your innovation strategically within the healthcare market. Engage doctors, decision-makers, and investors with a clear, evidence-based study strategy and communication.
Science on Demand
Access 20+ years of expertise in biomedical research, study strategy, and scientific copywriting directly within your team – with no overhead, no onboarding time, and immediate effectiveness.
We deliver measurable results- by advancing studies, driving revenue growth, and strengthening market presence.
We consult, write, coordinate, and fine-tune – with one goal: fast-tracking products to clinical relevance and market acceptance. Scientifically validated. Strategically minded.
Whether original clinical research
or review articles – we
design and produce
scientific content with
high impact. For life science
companies aiming to position themselves
at the forefront
of their field.
It is no coincidence,
that the best with us work.

Every successful life science company has these 3 secrets understood.
We are in the middle of a global innovation race. And it is not the best product that will prevail - but the company that most effectively combines scientific evidence, market access and communication.
Secret no. 1
Innovations don’t succeed on their own. Whether with KOLs, regulatory authorities, or investors – you need a robust study strategy and medical reasoning that builds trust before the competition hits the market.
Secret no. 2
Delays cost market share. Hitting milestones sooner means selling sooner – and standing out. Time-to-market (TTM) is the key lever on the road to clinical standard.
Secret no. 3
Medical innovations only earn credibility once their results are published – transparently, rigorously, and peer-reviewed. If it’s not published, it doesn’t count – neither in guidelines nor among KOLs. No publication strategy means no recognition, no success.
Most companies lose valuable time due to a lack of internal resources or medical,
regulatory, and commercial teams are not in sync – costing them their unique opportunity.
ABOUT THE COLLABORATION WITH US
Life science communication with clinical impact

As your external partner for scientific communication, publications and strategic medical positioning, we help you to make your clinical innovation visible more quickly - and to anchor it in the market in the long term.
Your advantages
Increase your Clinical Adoption Rate (CAR)
Reach your customers and the medical community effectively – with solid evidence, clear communication, and content that builds trust – so your product is adopted and recommended sooner.
Your innovation becomes visible faster
A professional study strategy combined with strategic communication accelerates the time it takes for your product to be recognized and understood by doctors, KOLs, investors, and the medical press.
Shorten your time to publication (TTP)
Excellent data - but no time or resources to publish it? We take care of the entire process, from manuscript preparation and submission to final acceptance, so that your results get to where they matter.
more Advantages
Instant Life Science Know-How
Bring 20 years of research, clinical, and industry experience directly into your team – no training, no friction, immediate impact. Leverage top-level know-how immediately.
Immediate relief for your team
Free up your teams to focus on operational priorities and strategic growth. We handle tasks for Medical Affairs, Clinical, Marketing/Sales, and Executive Management – efficiently and reliably.
Access to established networks & KOLs
Increase your presence in the medical community. We connect you with our extensive contacts in clinics, academia, and industry, both nationally and internationally.
Higher Approval and Adoption Rates
Your innovation receives the visibility and acceptance it deserves. We deliver robust evidence that counts in clinical guidelines, KOL discussions and approval processes.
Measurable Results, Delivered by Experts
Gain measurable, transparent outcomes. Our team of copywriters, statisticians, and marketing experts combines data-driven insights with strategic execution.
A strategic partner - not just a service provider
We provide ideas, feedback, and hands-on support to move your project forward with a clear, entrepreneurial perspective.
We Are Trusted by Leading Life Science Companies for These Services.
Testimonialsthat speak for themselves
Dr. Jörg Scheier
Vice President Medical Critical Care, CytoSorbents Europe GmbH
"Dr. Recknagel has been a key partner for us in scientific communication for many years. His ability to deal with medical and scientific content in a structured, comprehensible and highly professional manner makes him particularly valuable in our field of intensive care medicine and cardiac surgery. Whether in the preparation and publication of scientific data or in strategic coordination - he is a reliable and professionally strong partner who has supported us effectively in numerous projects and will continue to do so. Our long-standing cooperation with Dr. Recknagel is characterized by a high degree of trust, consistently high quality and professional sovereignty.
He understands our topics, our target groups - and always delivers exactly what we need. I can recommend working with Dr. Recknagel without reservation - professionally, strategically and personally."

Dr. med. Klaus Kogelmann
Former Chief Physician, Department of Anesthesiology and Intensive Care Medicine, Emden Hospital
"Dear Peter, it is a pleasure to write you a reference. In more than 10 years of scientific collaboration, Dr. Peter Recknagel was a congenial partner for me and always extremely helpful as my eyes and ears in our projects. We were able to publish our joint projects in a timely and targeted manner, with clear agreements on the roadmap and ultimately always at a high level. It is therefore a pleasure for me to enjoy this highly efficient and friendly collaboration and clearly recommend it to others."
Tobias Schwert
Therapy Development Manager, Heart Failure Department, Mechanical circulatory support
Abbott Medical GmbH
"Working with Peter is simply great. His quick understanding of different therapeutic approaches - including the respective technologies, indications and their limitations - is outstanding. In combination with his drive and efficiency, our joint projects (and there were several) were not only implemented at a high level, but were also a real pleasure."
The Management
Dr. Peter Recknagel
Founder & Managing Director
SCILS | Science Consulting in Life Science
Bringing more than 20 years of experience in research, clinical work, industry, and international consulting, Dr. Peter Recknagel contributes a broad scientific and strategic perspective to the life sciences sector. As biomedical scientist (PhD, Dr. rer. nat.) he has worked at leading research institutions such as University College London and Jena University Hospital in the areas of intensive care medicne, sepsis research, and molecular biomedicine.
Since 2012, he has been an independent consultant to medtech, pharma and biotech companies on clinically and scientifically sound market entries, medical affairs, study strategy and the publication of high-quality scientific content. Over 100 publications, case reports and specialist manuscripts are the result of his leadership or involvement.
Dr. Recknagel combines scientific excellence with entrepreneurial clarity. As a strategic sparring partner, he works at the interface between research, clinical practice and the market - for life science companies that not only want to impress with their products, but also set standards. "My job is to translate scientific depth into impact - in studies, communication and strategy."
The course of our cooperation:
OUR STRUCTURED 5-STEP PROCESS FOR SCIENTIFIC MARKET LEADERSHIP

STEP 1: STRATEGIC ONBOARDING
We start with a structured onboarding process: the aim is to clearly understand the product, clinical context, project goals and internal expectations. We use a short briefing format and coordinate directly with relevant departments such as Medical, Regulatory or Marketing.
➞ This creates a common understanding of content, tone and objectives right from the start.

STEP 2: ANALYSIS & TARGET GROUP FOCUS
We analyze the initial scientific situation, relevant studies and market requirements. At the same time, we sharpen the target group focus - be it for physicians, KOLs, investors, sales or regulatory authorities.
➞ The result: clear, target group-oriented communication that makes an impact.

STEP 3: RESEARCH & DATA PREPARATION
We research current literature, clinical guidelines, study results and relevant data. These are processed, contextualized and compared with existing statements.
➞ In this way, we ensure scientific substance, argumentative power and connectivity to current discourses.

STEP 4: CONCEPT & CONTENT IMPLEMENTATION
Based on strategy and data, we design content that is well-founded, understandable and effective. Whether study communication, specialist articles, white papers or training documents: the content follows the function.
➞ We formulate scientifically precise and at the same time target-oriented for clinical, regulatory and market-oriented contexts.

STEP 5: QUALITY CHECK & ON-TIME DELIVERY
Every piece of content goes through our structured quality control process. We check for scientific coherence, linguistic clarity, regulatory compliance and adherence to objectives.
➞ The end result is a reliable result that is publishable, citable and effective. On time. Without rework.
FAQ - YOU HAVE OPEN QUESTIONS?
What exactly does SCILS do?
SCILS specializes in scientific-strategic consulting and communication for companies in the life sciences sector. We support our clients in study strategy, publication planning, medical copywriting and scientifically sound market communication - with the aim of establishing medical innovations on the market faster and more convincingly.
For whom does the collaboration make sense?
SCILS specializes in scientific-strategic consulting and communication for companies in the life sciences sector. We support our clients in study strategy, publication planning, medical copywriting and scientifically sound market communication - with the aim of establishing medical innovations on the market faster and more convincingly.
What kind of projects does SCILS take on?
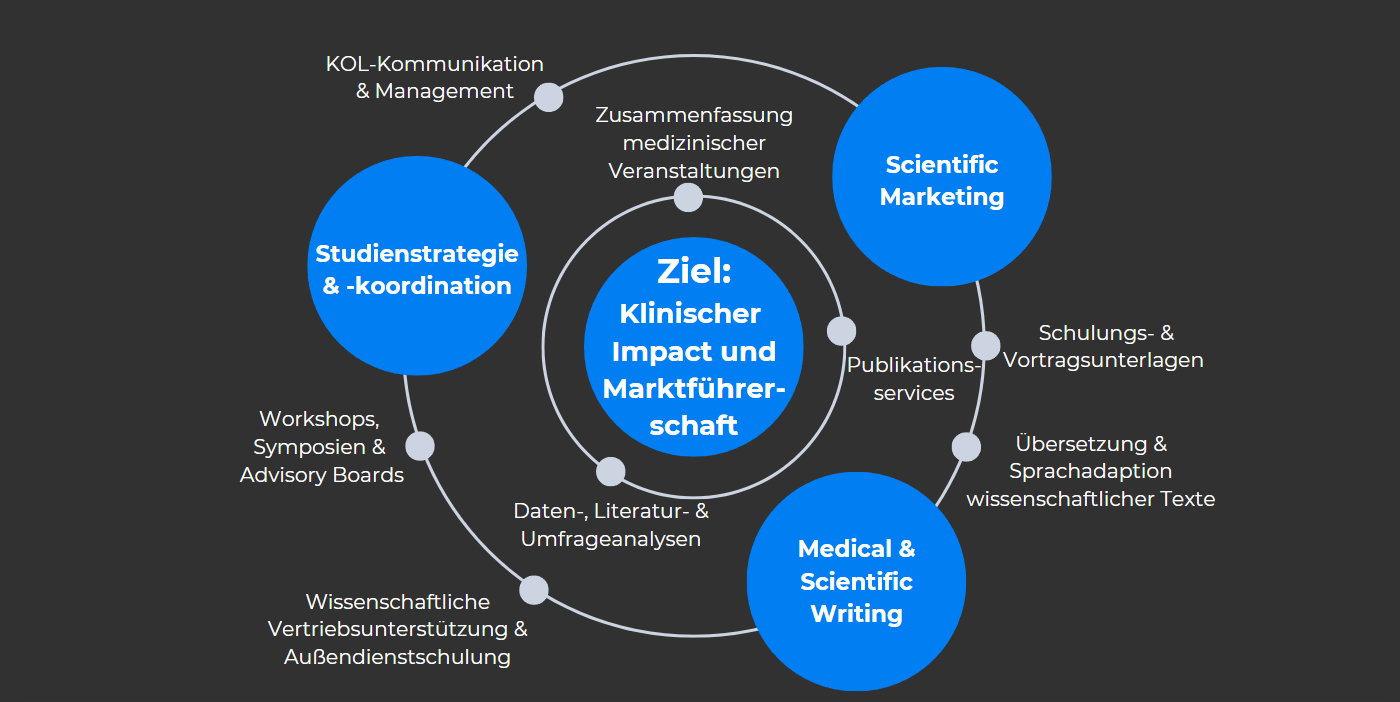
Among other things, we look after
- Study strategy & study support: Development, initiation and coordination of national and international studies - both clinical and basic scientific.
- Scientific Marketing & Evidence Analysis: Research, evaluation and strategic preparation of scientific data for targeted product positioning
- Medical & Scientific Writing: Preparation, revision and publication of case reports, studies, reviews - including monitoring of the peer review process through to final publication
- Training & presentation content: Conception and design of high-quality lectures, training materials and medical sales presentations
- Target group work & communication: Scientific support for sales teams, training, workshops and cooperation with KOLs and advisory boards
- Data, literature & survey work: conducting and evaluating medical research and surveys - as a basis for communication, studies or market analysis
- Technical documentation & meeting support: creation of meeting minutes, summaries of symposia and scientific events
- Translations & language adaptations: Professional and precise translation of scientific content (German-English / English-German) for international visibility.
How does the collaboration work?
What results can I expect?
You receive reliable, scientifically sound content - precisely formulated, publishable at a high level and effective in the market. Whether publication, presentation or study communication: impact is our goal.
How confidential is the collaboration?
Absolutely confidential. We have been working with sensitive, project-related data for many years. Of course, we offer an NDA or individual confidentiality agreement on request.
How long does a project take?
This depends on the scope. We usually deliver shorter text formats (e.g. white papers, abstracts) in 1-2 weeks. Extensive study or publication projects take correspondingly longer - always with a clear timeline and punctual delivery.